Welcome to my beginner's guide to Surface Tension, Surfactants, and Micelles. In this blog, we'll be exploring the basics of these topics in order to better understand their role in industry and everyday life. Surface tension is a crucial property of a liquid that allows it to resist being pulled apart by gravity, and surfactants are molecules that can lower the surface tension of water. Micelles are spherical structures that are formed when surfactants reach a certain concentration in water, and they play an important role in many cleaning and industrial processes. We'll be discussing all of this and more in this blog, so let's get started!
Surface Tension Overview
Surface tension is a property of liquids that refers to the cohesive forces between molecules at the surface of the liquid. These forces cause the surface of the liquid to act like a  thin, elastic membrane. Surface tension is what allows insects to walk on water, and it also plays an important role in many industrial processes. Surface tension is measured using a tensiometer. These tensiometers use a platinum ring (duNouy Ring) or a flat plate (Wilhelmy Plate) to detect the strength of surface tension.
thin, elastic membrane. Surface tension is what allows insects to walk on water, and it also plays an important role in many industrial processes. Surface tension is measured using a tensiometer. These tensiometers use a platinum ring (duNouy Ring) or a flat plate (Wilhelmy Plate) to detect the strength of surface tension.
The Role of Surfactants in Surface Tension
Surfactants are chemicals that lower the surface tension of a liquid. They do this by adsorbing at the air-water interface and forming a monolayer. This monolayer lowers the cohesive forces between liquid's molecules, and as a result, the surface tension is lowered. The most common surfactant is soap, which is used in many cleaning and industrial processes. Other surfactants include detergents, cosmetics, paints, and coatings.
The Role of Micelles in Surfactant Systems
When surfactants reach a certain concentration (known as the critical micelle concentration, or CMC), they begin to f orm micelles. Micelles are spherical structures with the hydrophobic tails of the surfactant molecules pointing inward and the hydrophilic heads pointing outward. This structure allows them to trap hydrophobic contaminants (like dirt and oil) in the center of the micelle, where they can then be removed by the carrier liquid. Micelles play an important role in many cleaning and industrial processes, as they are very effective at removing hydrophobic contaminants.
orm micelles. Micelles are spherical structures with the hydrophobic tails of the surfactant molecules pointing inward and the hydrophilic heads pointing outward. This structure allows them to trap hydrophobic contaminants (like dirt and oil) in the center of the micelle, where they can then be removed by the carrier liquid. Micelles play an important role in many cleaning and industrial processes, as they are very effective at removing hydrophobic contaminants.
Surface Tension Applications
Surface tension is a very important property of liquids, and as such, it has many applications in industry and everyday life. For example, surface tension is used in the manufacturing of paper and textiles. It is also used in the printing industry, as it allows inks to stick to surfaces. Surface tension is also responsible for the shape of water droplets, and it plays a role in the function of many biological systems.
Surfactant Applications
Surfactants are used in many different industries and applications. They are used in the cosmetics industry to lower the surface tension of water, which allows for a more uniform application of makeup. 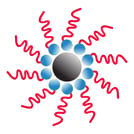 They are also used in the cleaning industry, as they are very effective at removing hydrophobic contaminants. In addition, surfactants are used in the food industry to emulsify fats and oils, and they are also used in the pharmaceutical industry. Surface tension, surfactants and micelles are all important concepts to understand if you want to know how many industrial processes work. They also have many everyday applications, from the makeup we put on our faces to the food we eat. So next time you see a water droplet or use soap, remember surface tension is what makes it possible and micelles do the work!
They are also used in the cleaning industry, as they are very effective at removing hydrophobic contaminants. In addition, surfactants are used in the food industry to emulsify fats and oils, and they are also used in the pharmaceutical industry. Surface tension, surfactants and micelles are all important concepts to understand if you want to know how many industrial processes work. They also have many everyday applications, from the makeup we put on our faces to the food we eat. So next time you see a water droplet or use soap, remember surface tension is what makes it possible and micelles do the work!
If you find this useful, please forward a copy to colleagues who have similar interests.
After this, I lowered my level of mystification about CMC but it is still somewhat edgy.
Art
P.S. Subscribe, at the top of this page, to keep up with these excursions through the world of test equipment.
P.P.S. CSC Scientific provides tensiometers that range from classical manual operation to fully automated instruments that perform 10 additional surface tension related measurements including Critical Micelle Concentration (CMC). Take a look at this unique selection by clicking on the image.


