Surface Tension
There are many ways that surface tension is defined. The definitions range from very simple such as:
Surface Tension is water's ability to "stick to itself".
To a more technical definition that states:
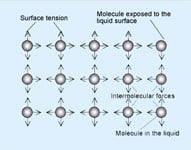 A liquid exists as a liquid because of the attractive forces between molecules. These are called intermolecular forces, or van der Waals' forces.
A liquid exists as a liquid because of the attractive forces between molecules. These are called intermolecular forces, or van der Waals' forces.
Molecules within the liquid are surrounded by other molecules and are attracted in every direction with equal force. Molecules exposed to the surface are unstable because the attractive forces are not equal and they are drawn away from the surface. As a result the liquid tends to contract the surface area until equilibrium is reached. That happens when the surface reaches its minimum.
These intermolecular forces which contract the surface are called "Surface Tension".
There are many conditions that affect the Surface Tension (ST) of liquids. 
- Temperature - Increasing temperature lowers ST
- Surfactant - Surface acting agents lower ST
- Impurities - Change ST
- Oxidation - Changes ST
- Chemical Quality - Detected by different ST
The measurement of Surface Tension is important in the development and quality control of such things as ink, soap and detergent, pharmaceutical compounds, adhesives and a wide range of coatings.
There are several methods used for measuring Surface Tension. The most common is a technique developed by P. Lecomite duNouy and popularized in a paper published in 1925. The technique has become known as the duNouy Ring Method. CSC uses this method in its line of Tensiometers. Further, there is a technique called the Wilhelmy Plate Method. This method is included in the DY Series Automatic tensiometers.

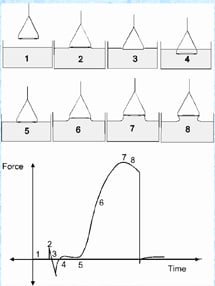

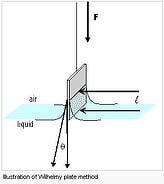
 We offer the
We offer the  The instrument offers duNouy ring and Wilhelmy plate methods of automatically measuring and calculating actual surface tension, no correction calculation required.
The instrument offers duNouy ring and Wilhelmy plate methods of automatically measuring and calculating actual surface tension, no correction calculation required. 
