One of the ways to describe surface tension in fluids is: the property of a liquid’s surface that resists force. It serves as a barrier to foreign materials and holds the liquid together. This ever-present property is caused by unbalanced forces on surface molecules that pull toward the main part of the liquid.
What are some of the primary conditions that affect surface tension? The surface tension
characteristics of a fluidic substance stay basically stable,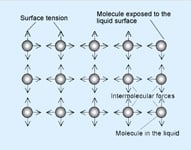 but can be changed by temperature variations, chemicals that modify the bonding characteristics of the molecules, oxidation and the presence of impurities. Let’s consider the effects of these four conditions on surface tension:
but can be changed by temperature variations, chemicals that modify the bonding characteristics of the molecules, oxidation and the presence of impurities. Let’s consider the effects of these four conditions on surface tension:
Temperature
As temperature decreases, surface tension increases. Conversely, as temperature increases, surface tension decreases becoming zero at its boiling point.
Chemical Additions
.jpg?width=80&height=80&name=chemical-impurities_(1).jpg) Adding chemicals to a liquid will change its surface tension characteristics. The effect of adding an unrelated chemical to a substance, and thereby changing its surface tension, is demonstrated by the example of putting soap (a surfactant) in water to reduce the surface tension, which allows the dirt on your hands to more easily mix with the water.
Adding chemicals to a liquid will change its surface tension characteristics. The effect of adding an unrelated chemical to a substance, and thereby changing its surface tension, is demonstrated by the example of putting soap (a surfactant) in water to reduce the surface tension, which allows the dirt on your hands to more easily mix with the water.
Oxidation
Oxidation directly affects surface tension. As surface tension increases, intermolecular forces increase. Oxygen in the atmosphere is known to decrease the surface tension of various substances. The Presence of ImpuritiesThe presence of impurities on the surface of, or dissolved in, a substance directly affects the surface tension of the liquid. The surface tension of water, for example, will increase when highly soluble impurities are added to it.
Surfactant
Now that we've considered the effects of variation in temperature, the addition of chemicals, oxidation, and the presence of impurities on surface tension, maybe we should next consider the effect of a surfactant over time and how we observe and measure this effect.
The subject of conditions affecting surface tension appears at this level to be quite easy to understand. I am sure that as soon as I feel comfortable with this idea, I'll get a question that rattles me again.
I remain a disconcerted,
Art
P.S. If you are not subscribed to these Test Equipment rants and muses, sign up at the top of the page.
Image via Wikimedia


