One quiet night, I was musing over moisture analysis and how easy it is to do using loss-on drying. Little did I know what was in store.
Of course, everybody knows that all you do is 1. weigh a sample, 2. dry it out and then 3. weigh it again. However, I decided that a modicum of research about drying would be prudent before I started. As I got into it, this small inquiry into moisture testing expanded into a lot more about evaporation, vapor pressure, water content complexities -- and the spookiest of the bunch, water activity -- all just to determine the effects of drying something.
I decided that a modicum of research about drying would be prudent before I started. As I got into it, this small inquiry into moisture testing expanded into a lot more about evaporation, vapor pressure, water content complexities -- and the spookiest of the bunch, water activity -- all just to determine the effects of drying something.
Do I talk about it all at once or break it into individual mysteries? I found the former approach overwhelming, but with respect to the latter --- Where to start?
After brooding for a while, I concluded that evaporation was important to the loss-on drying process; simple in concept and easy to understand. Consequently, I start with evaporation because it is elementary -- if you don't consider the influence of vapor pressure, atmospheric pressure, temperate and humidity.
drying process; simple in concept and easy to understand. Consequently, I start with evaporation because it is elementary -- if you don't consider the influence of vapor pressure, atmospheric pressure, temperate and humidity.
It seems that evaporation is all about water molecules bumping into each other. If they are highly agitated by an energy source, they fly away from their neighbors and transform a closely packed liquid into a loosely packed 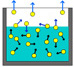 gas or vapor. At various times, the flow balances; some switch to vapor, some switch back to liquid (or as we know it, water). Changing the ambient pressure, temperature or humidity changes this balance, requiring more or less energy to affect the evaporation volume.
gas or vapor. At various times, the flow balances; some switch to vapor, some switch back to liquid (or as we know it, water). Changing the ambient pressure, temperature or humidity changes this balance, requiring more or less energy to affect the evaporation volume.
When things settle into equilibrium, the gas  molecules sit at the liquid surface and exchange positions with the liquid molecules. At this gas-liquid boundary, vapor pressure increases with heat and decreases if cooled. The more the vapor pressure, the more flying gas molecules are generated, thus resulting in more evaporation.
molecules sit at the liquid surface and exchange positions with the liquid molecules. At this gas-liquid boundary, vapor pressure increases with heat and decreases if cooled. The more the vapor pressure, the more flying gas molecules are generated, thus resulting in more evaporation.
The energy, usually heat, gets the highly agitated molecules to fly out of the liquid. These hot ones leave behind the cooler ones, cooling the liquid. This is how perspiration evaporating from your brow cools you on a hot, dry summer day.
liquid. These hot ones leave behind the cooler ones, cooling the liquid. This is how perspiration evaporating from your brow cools you on a hot, dry summer day.
All of these influence loss-on drying tests. I thought it was “just turn on the heat and wait;” -- No considerations of bouncing molecules, summer or winter, clear or stormy weather to confuse and change evaporation.
Now we know some of the reasons that loss-on drying tests sometimes take longer or shorter than normal periods.
Maybe, someday I'll get the courage to take on vapor pressure, water content, drying and even water activity. Look for it.
Thanks for reading this rant, hope it was amusing and maybe helpful.
As always, a mystified,
Art
P.S. Sign up to subscribe to these test equipment rants, news spots and background notes by filling in your email in the subscribe blank in the upper right.
P.P.S. See where Loss On Drying works best


